OEM&ODM Service
• According to your design we can be your workshop.Take advantage of close cooperation with moulding factory ,skilled worker and advaced equipment and own test lab,we can offer.
• Specific designs and material combinations.
• Solutions adapted to current and future isolation technologies for aseptic filling.
• Cross-functional flexible Project Management.
• Solutions that comply with the latest regulatory requirements.
One Stop Trading Service
• As friend company of many other pharmaceutical packaging companies, we are good business partner with pharmaceutical glass vials,flip off caps,and pefilled syringe companies in China.
Lab Service
• Analysis for the characterization of extractables , leachables and other chemical properties.
• Extractable and toxicology characterization packages ready for submission to the other regulatory bodies.
• Tomography used as a non-destructive tool to evaluate packaging systems, drug delivery devices and final drug products.
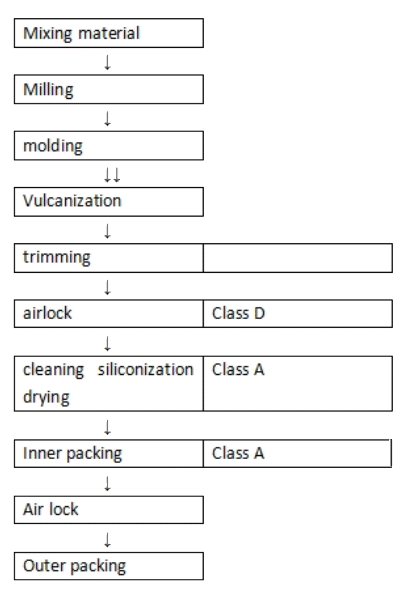
Flow Chart
Laboratory Photos


